How Many Valence Electrons Does Roentgenium Have
The last shell of chromium has one electron and the d-orbital has a total of five electrons3d5. 113 Nh - Nihonium.
Roentgenium Valence Electrons Roentgenium Valency Rg Dot Diagram
111 Rg - Roentgenium.

. Crystal structure of Roentgenium. Roentgenium is a chemical element with atomic number 111 which means there are 111 protons and 111 electrons in the atomic structure. Therefore the number of electrons in neutral atom of Roentgenium is 111.
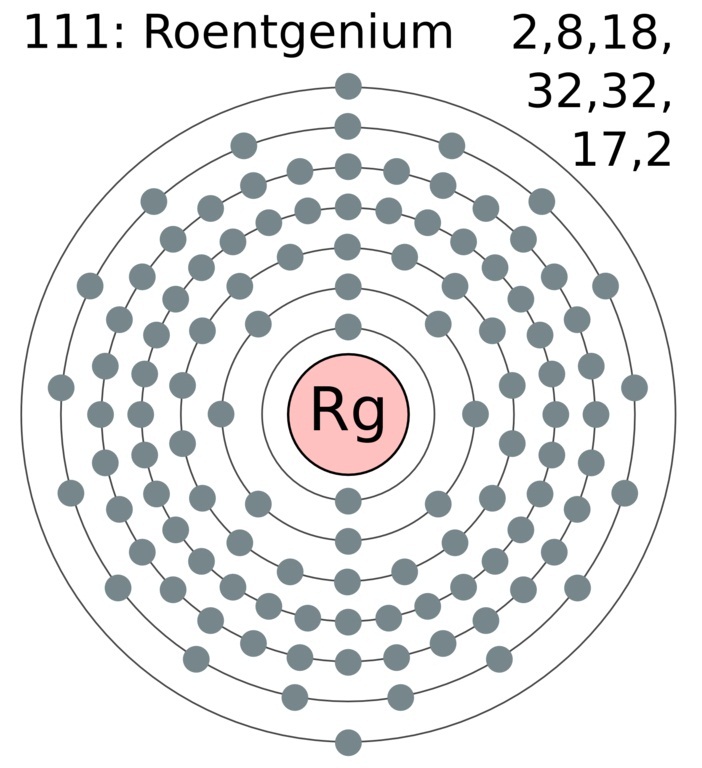
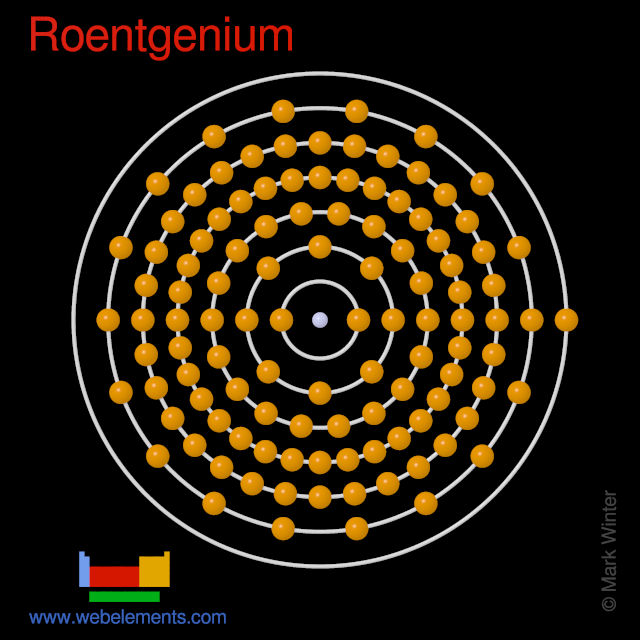
Therefore the valence electrons of chromium are six. Roentgenium atoms have 111 electrons and the electronic shell structure is 2 8 18 32 32 17 2. There is an easy path to find valence electron which are located in the last shell of Sulfur.
3080 kJmol all estimated Is roentgenium a solid liquid or gas. 2 8 18 32 32 17 2. And based in the oxidations states of other member of its group Group 11 the common oxidation states of roentgenium include 5 and 3.
18 4-st level N. Number of neutrons Atomic mass of a given isotope of Rg - 111. Each electron is influenced by the electric fields produced by the positive nuclear charge and the other Z 1 negative electrons in the atom.
One would predict its behaviour to be similar to that of gold immediately above roentgenium in the periodic table and silver two places above. So Sulfur valence electrons are six. Each electron is influenced by the electric fields produced by the positive nuclear charge and the other Z 1 negative electrons in the atom.
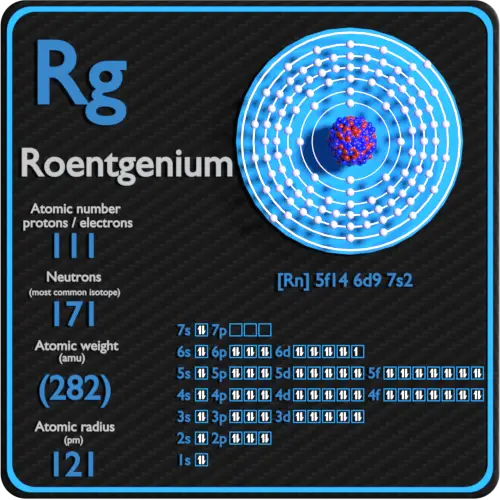
Electron Configuration and Oxidation States of Roentgenium. Atomic mass of Roentgenium most stable isotope 282 u. 112 Cn - Copernicium.
Electrons arrangement in Roentgenium or Bohr model of Roentgenium. The atomic number of Arsenic is 33. Roentgenium has 111 electrons.
Electronic configuration of the Roentgenium atom. It has similar reactivity as gold but is thought to produce more stable and. Valence electrons are the electrons in the outermost shell of an atom.
The atomic number of each element increases by one reading from left to right. Distribution of electrons over energy levels in the Rg atom 1-st level K. 2 8 18 32 32 17 2 predicted What is the electron configuration for roentgenium.
Roentgenium has 111 protons an 111 electrons. Roentgenium is a chemical element with atomic number 111 which means there are 111 protons and 111 electrons in the atomic structure. Electrons and Electron Configuration.
The number of electrons in an electrically-neutral atom is the same as the number of protons in the nucleus. How many electrons does roentgenium have. For atoms with many electrons this notation can become lengthy and so an abbreviated notation is usedThis is important as it is the Valence electrons 5f14 6d10 7s1 electrons in the outermost shell that determine the chemical properties of.
Roentgenium is a chemical element with symbol Rg and atomic number 111. Possible oxidation states are. How many protons neutrons and electrons are in a roentgenium atom.
Members of a group typically have similar properties and electron configurations in their outer shell. Electron configuration of Roentgenium is Rn 5f14 6d9 7s2. Roentgenium electron configuration Electronic configurations of elements.
32 5-st level O. Rn 5f 14 6d 9 7s 2 predicted Roentgenium ionization energies. The chemical symbol for Roentgenium is Rg.
It has been predicted to be a Nobel element. 8 3-st level M. A vertical column in the periodic table.
The chemical characteristics of roentgenium have not been studied in detail yet 2. Reaction of roentgenium with bases. Properties of Roentgenium Atomic Mass of Roentgenium Atomic mass of Roentgenium is 272 u.
As only a few atoms of roentgenium have ever been made its reactivity with bases is unknown. The standard electrode potential of 19 V for the Rg 3 Rg couple is greater than that of 15 V for the Au 3 Au couple. Therefore the number of electrons in neutral atom of Roentgenium is 111.
Classified as a transition metal Roentgenium is a expected to be a solid at room temperature. It means that Sulfur atom has 16 protons and 16 electrons. The electrons in the last shell is equal to sulfur S valence electrons.
The chemical symbol for Roentgenium is Rg. Therefore the order of the number of electrons in each shell of the roentgenium Rg atom is 2 8 18 32 32 17 2. The number of electrons in an electrically-neutral atom is the same as the number of protons in the nucleus.
We have shown the Valence Electrons of the elements for which reliable data is available. The easy way is to write the electron configuration. As Arsenic.
Roentgenium Rg electron configuration Bohr model The fifth shell of roentgenium will have thirty-two electrons seventeen electrons in the sixth shell and the remaining two electrons will be in the seventh shell. Electronic configuration of Roentgenium Rn 5f 14 6d 9 7s 2. 2 2-st level L.
Neutrons in Roentgenium. Roentgenium is predicted to be a noble metal. A horizontal row in the periodic table.
Valence Electrons Graph - Valence Electrons of all the elements in graph. Roentgenium Properties See also.

Roentgenium Atomic Structure Stock Image C018 3792 Science Photo Library

Roentgenium Electron Configuration Symbol Atomic Number Atomic Mass Oxidation States Standard State Group Block Year Discovered
Webelements Periodic Table Roentgenium Properties Of Free Atoms
Roentgenium Protons Neutrons Electrons Electron Configuration
No comments for "How Many Valence Electrons Does Roentgenium Have"
Post a Comment